http://www.kurzweilai.net/intelligent-molecules
 It sounds like science fiction: “intelligent molecules” that react to external stimuli and reversibly change their shape.
It sounds like science fiction: “intelligent molecules” that react to external stimuli and reversibly change their shape.
But now Ludwig-Maximilians-Universität (LMU) physicists have succeeded for the first time in creating a chemical reaction, using a single polymer molecule, that makes this process visible.
 Dr. Michael Nash
and his colleagues placed a self-generated synthesized polymer on a
gold surface using an atomic force microscope (AFM). One polymer end
adhered to the surface and the other end to the tip of the AFM.
Dr. Michael Nash
and his colleagues placed a self-generated synthesized polymer on a
gold surface using an atomic force microscope (AFM). One polymer end
adhered to the surface and the other end to the tip of the AFM.
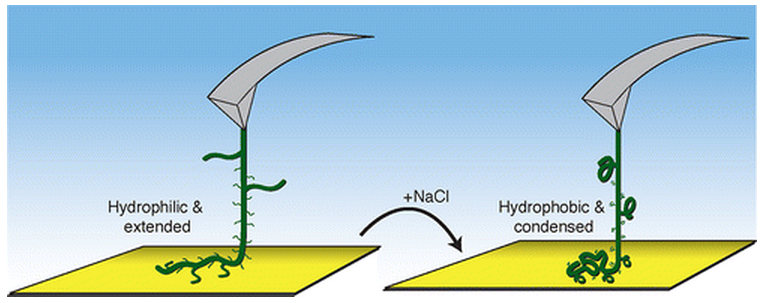
Once the scientists increased the salt concentration of the surrounding medium, they observed that the molecule collapsed gradually.
“In a highly concentrated salt solution, the polymer compound dehydrates and shrinks,” says Nash. “Back in a weak salt solution, the molecule unfolds again.
“We have observed both processes in our study for the first time for a single polymer molecule.”
The new method could provide an important element for nanoswitches of the future and could also be used in biosensors, drugs, chromatography procedures, and other applications, the researchers suggest.
Dr. Michael Nash is with the Prof. Hermann Gaub group, a member of The Cluster of Excellence Nanosystems Initiative Munich (NIM),
Intelligent molecules
January 16, 2013

Solvent-induced
collapse of an environmentally responsive copolymer (attached to a gold
surface and an atomic force microscope tip). The molecule changes
length in response to increased salt concentration. (Credit: Michael A.
Nash, and Hermann E. Gaub/ACS Nano)
But now Ludwig-Maximilians-Universität (LMU) physicists have succeeded for the first time in creating a chemical reaction, using a single polymer molecule, that makes this process visible.

(Credit: Christoph Hohmann)
Once the scientists increased the salt concentration of the surrounding medium, they observed that the molecule collapsed gradually.
“In a highly concentrated salt solution, the polymer compound dehydrates and shrinks,” says Nash. “Back in a weak salt solution, the molecule unfolds again.
“We have observed both processes in our study for the first time for a single polymer molecule.”
The new method could provide an important element for nanoswitches of the future and could also be used in biosensors, drugs, chromatography procedures, and other applications, the researchers suggest.
Dr. Michael Nash is with the Prof. Hermann Gaub group, a member of The Cluster of Excellence Nanosystems Initiative Munich (NIM),



No comments:
Post a Comment